Guides
Register for an Account
AlzPED can be searched without registering for an account. To submit your study data, you need an AlzPED account.
Review detailed steps for the full process and then begin with : Create an Account
- Select “Create an account” from above.
- Enter your Username, E-mail Address, Password, and confirm your Password.
- Provide brief account information: First Name, Last Name, Middle Initial, Organization Name, and your Phone Number.
- Answer the Captcha challenge.
- Click on “Create New Account”.
- You will receive a confirmation message on the main AlzPED showing the account is pending approval.
- You will also receive a confirmation message: Account details for Your AlzPEDaccount at AlzPED (pending System Administrator approval).
- Once approved a second e-mail message: Account detail for YourAlzPEDaccount at AlzPED (approved), will arrive.
- Click on the link within the e-mail message to get to the Login page.
- YOU WILL NEED TO REMEMBER YOUR PASSWORD, as no one will have access to that information.
- Enter your Username or E-mail address and your password.
- Click on Log In
* If you do not receive a confirmation message, please Contact Us.
* If you should forget your password please use the “Reset Password” instructions. This will bring you to a screen with your login information.
You are ready to proceed to “Submit Your Data” to enter your study information.
Search Guide
AlzPED can be queried in a variety of ways including a general text search or searching individual fields such as: Title, PI Name, Primary Reference (PubMed ID), Funding Source, Therapy Type, Therapeutic Target, Therapeutic Agent, Model Name and Model Type.
A general text search can be performed from the main “Search” box on the home page or the Full Text Search box found by using the “Search AlzPED” link on the Home page, under the AlzPED banner.
Text queries search the entire textual content of all fields of the database and, links to other databases (e.g., related citations in PubMED, Clinical trials.gov, patents). Using a general text query you can search the database for therapeutic targets (e.g., “beta-secretase”,BACE1, “gamma-secretase”, “HMG-CoA reductase” ), therapeutic agents (e.g., curcumin, simvastatin, bexarotene ), animal models (e.g., Tg2576, 3xTg).
Text term queries are not case sensitive however, text terms/phrases should be placed in quotes, (“”) so the system knows to search for that exact character string. Otherwise the search engine will search for each term in the phrase individually. For example, compare searches with:
“beta secretase” - yielding 13 results vs. beta secretase - yielding 28 results
In the case of the latter the search engine will search for beta OR secretase.
If two terms are entered into a single field the default Boolean operator is OR.
If terms are entered into separate fields the default Boolean operator is AND.
* Note that more accurate search results are likely to be obtained by searching the individual fields as described below.
Individual fields - PI (Principal Investigator) Name, Title, PubMed ID, Funding Source, Therapy Type, Therapeutic Agent, Therapeutic Target, Animal Model Type and Animal Model Name can be searched by selecting either the “Search AlzPED” link under the AlzPED banner, or selecting “More Search Options” directly under the main Search Box on the Home page.
BIBLIOGRAPHIC
Full Text Search: This search box performs identically as the main Search box on the Home page.
Title: Keywords, phrases, and full-titles can be searched in this field. Place quotes (“”) around key words and phrases, to search for a specific string of characters. Otherwise the system will search for each term within the phrase or title individually, producing a much larger result set. If two terms are entered into a single field the default Boolean operator is OR. If terms are entered into separate fields the default Boolean operator is AND.
Primary Reference (PubMed ID): Enter the PubMed ID number to find AlzPED information related to articles indexed in Medline PubMed. For published papers, please see the original publication or PubMed.gov to identify the PMID and search by the PMID.
Contact PI/Author Name: Authors can be found by searching either their last name, full name or a variation of those names. *Note: Only the identified Corresponding Author has been entered into the system; co-authors will not be found.
Funding Source: Funding Organizations can be searched by beginning to type the full name of the funding organization, and then using the drop down menu to select the organization. Multiple organizations can be selected by holding down the Control key.
THERAPEUTIC AGENT
Therapy Type: Refers to the agent classification based on source (natural product or synthetic), molecular structure (small molecule, biologic), chemical nature (protein, peptide, hormone, RNA, DNA), mechanism of action ( immunotherapy active, immunotherapy passive).These can be queried by clicking in the Therapy Type field and choosing an option. Multiple Therapy Types can be selected by holding down the control key and selecting additional Therapy Types. Alternatively, you can continue searching by typing and selecting other choices from the drop down menu as well.
Therapeutic Target: Refers to the molecular or cellular structure through which an agent mediates its therapeutic activities. AlzPED lists targets alphabetically; these can be searched by clicking in the Therapeutic Target field and choosing one of the target options or just by beginning to type the target name. Multiple targets can be selected by continuing this process until you are ready to execute the search.
Therapeutic Agent: Users can find agent entries by typing the agent name in the Therapeutic Agent field. A query can contain more than one agent name (e.g., Tadalafil, Sildenafil).
ANIMAL MODELS
Model Name: Refers to the designation given to the animal model by the scientific community. AlzPED houses the names of large number of animal model names including transgenic mice and rats, non-trangenic mice and senescence-accelerated mice. Also housed are names of “wild-type” animals used in therapy development (e.g., aged mice, young and aged Fischer, Wistar, Sprague-Dawley rats, Rhesus and Cynomolgous non-human primates, etc). To search start typing in the Model Name field and options will appear in the drop down menu. Multiple model names can be selected for a single search by selecting multiple options from the drop down menu.
Model Type: Refers to the human gene of homolog associated with neuropathological and clinical phenotypic characteristics of Alzheimer’s disease. Model types can include single or multiple combinations of genes (e.g., APP, ApoE4, APPxPS1, hTau, APPxPS1xhTau, etc). Excluded are animals with genetic manipulations that have limited clinical relevance to AD (e.g., knock-outs of APP, tau, PSEN1 and PSEN2). To query model types start typing in the Model Type field and select options from the drop down menu. Select multiple options by using the control key.
SEARCH RESULTS
General text or individual field searches will take the user to the Search Results screen. There each record is displayed by AlzPED ID (APID), Title, Year, Contact PI and Therapeutic Agent. To view the data for an individual record click on the title. Records can be sorted by APID, Year or Contact PI. Results can be exported to Excel using either the All Results or Selected Results options at the top of the Search Results screen.
How to Enter Data
AlzPED is designed as a knowledge platform for the housing, sharing and mining of both published and preprint data relating to the preclinical testing of candidate therapeutic agents in AD animal models. The data housed in AlzPED are gleaned from two sources – 1) the scientific literature and 2) directly from researchers and, submitted through a curator. Once registered AlzPED users can clickon Submit Your Data and upload published or, preprint data. For direct submission researchers must register their studies on line and submit data directly. In this context data refers to information about a study, not the scientific data collected during the study. The curator will conduct the initial review and determine if the study is acceptable for incorporation into the database.
MINIMUM REQUIREMENTS FOR A SUBMITTED STUDY TO BE CONSIDERED FOR REVIEW AND PUBLICATION:
1. All sections must be completed.
2. The study must include information on at least one Therapeutic Agent and Animal Model. Each field in the Therapeutic Agent and Animal Model sections must be completed.
3. Review the Experimental Design section carefully and select all areas of Experimental Design for which you have information to report for the study.
To enter Published Studies go to PUBLISHED STUDY - How to Enter Data.
To enter Preprint Studies go to PREPRINT STUDY - How to Enter Data.
You may save information within each section (complete or incomplete), to move onto the next section. You can return to the previous sections any time before you upload the data to AlzPED.
To assist with data entry please follow the tool tips (?) provided in each section.
If you have questions please Contact Us.
How AlzPED Citations are Selected
AlzPED is intended to include efficacy studies from published and preprint reports. Published studies for AlzPED have been gleaned from databases including: Medline PubMed, EMBASE, ISI Web of Science, and SCOPUS.
Characteristics of Studies Selected for Inclusion
All of the published studies selected for this database must:
- Be written in English
- Have been published between 1995 and the present
- Test one or more therapeutic agents (excluding those that are non-pharmacological; e.g., exercise, environmental enrichment, diet, genetic etc.)
- Test therapeutic agents in: APP, Tau and Presenlin- based transgenic rodent models; rodent models based on beta amyloid injection; aged rodent models; senescence accelerated rodent models; aged canine models, non- human primate models. Genetic manipulations such as knock-outs of APP, Presenilin, Tau, ApoE, etc, are not included since they have no relevance to clinical Alzheimer’s disease.
- Evaluate efficacy of treatment on the Alzheimer's disease phenotype exhibited by at least one of the above listed models
- Provide methods, results, outcomes, and conclusions from the study
Terms/Concepts Used for Identifying the Literature
Search terms and concepts for the databases and journals mentioned above are intended to capture an inclusive result set as possible, while still producing a successful result set. Three concepts have been included in the search strategies:
Search Concept A: Alzheimer’s, Alzheimer’s disease, Tau, Beta Amyloid Plaque, Cognitive Memory Impairment, and Dementia
Search Concept B: Animal Model, Mouse, Mice, Rodent, Rat, Canine, Dog, Chimpanzee, Chimp, and Non-human primate
Search Concept C: Therapy, Therapeutics, Treatment, Efficacy, Efficacious, and Drug development
PUBLISHED STUDY - How to Enter Data
MINIMUM REQUIREMENTS FOR A SUBMITTED STUDY TO BE CONSIDERED FOR REVIEW AND PUBLICATION:
1. All sections must be completed.
2. The study must include information on at least one Therapeutic Agent and Animal Model. Each field in the Therapeutic Agent and Animal Model sections must be completed.
3. Review the Experimental Design section carefully and select all areas of Experimental Design for which you have information to report for the study.
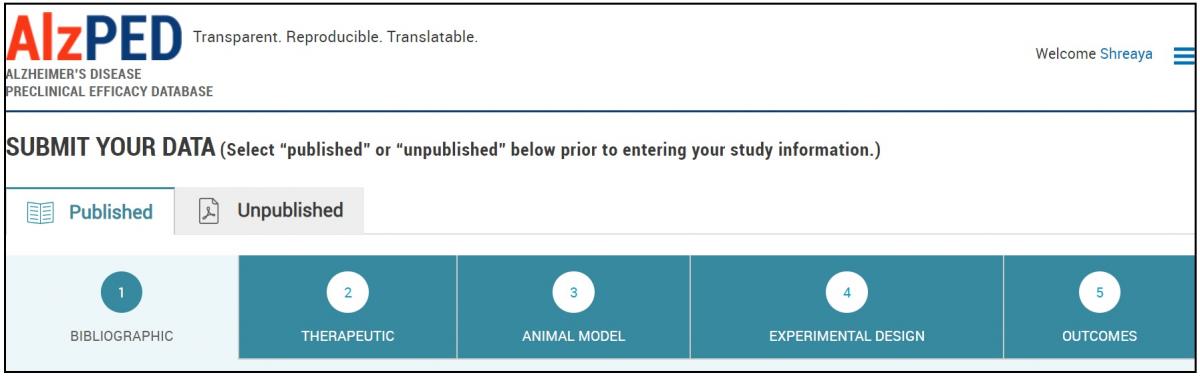
HOW TO ENTER DATA
Select the “Published” tab and complete the 5 data sections: BIBLIOGRAPHIC, THERAPEUTIC, ANIMAL MODEL, EXPERIMENTAL DESIGN and OUTCOMES, following the detailed instructions provided for each section. For explanations about the fields in each section follow the provided Tool Tips (?).
You may save information within each section (complete or incomplete), to move onto the next section. You can return to the previous section any time before you upload your data to AlzPED. Please note: once you submit your data, you cannot make changes until the curator has reviewed the citation. The curator will contact you with any questions or concerns, prior to adding the citation to the AlzPED database. If you have any questions, please Contact Us.
SECTION 1 – BIBLIOGRAPHIC
- Complete the fields in this section shown below. Steps 2 – 12 provide detailed instructions about each field.
- Year of Publication: Select the year the research article was published from the drop down menu.
- Title of Study: Enter the full title of the research article. Click on the “Show Special Characters” link for a list of special characters to include in the title, if required. Contact us if you want to use characters not shown in this tool.
- Contact PI Information – Last Name, First Name, Middle Initial: Enter the name of the primary point of contact (corresponding or senior author) for the research article. If the contact PI formally goes by a middle name, then an initial can be entered in the “First Name” field and a full name can be entered in the “Middle Initial” field.
- Contact PI Affiliation: Enter the Department, School, Institution, City, Region or State, and Country for the contact PI.
- Co-Authors: Enter the names of all co-authors for the research article.
- Primary Reference (PubMed ID/PMID): This is the 8 digit numerical ID assigned to a published study in PubMed. Enter this numerical ID in this field or leave this field blank and it will be completed by the AlzPED Curator.
- Funding Source: Enter any financial sources that supported the research. Select one or more funding sources from the drop down menu or enter the funding source if it does not appear within the list.
- Study Goal and Principal Findings: Enter the primary study goal(s), and the overall outcomes found, and the information learned from the study. You can enter your research article abstract in this space.
- Bibliographic Notes: Enter any unique features regarding the citation or bibliographic information. For example, this space can be used to specify the names of co-authors who have made equal contributions to the study or list additional corresponding authors or co-investigators.
- Save this section. You will not be able to access other sections until you save the information you have entered in this section.
- Click the “Next” button to move on to the next section. Alternatively, you can complete the remaining sections in any order by clicking on the tab for that section.
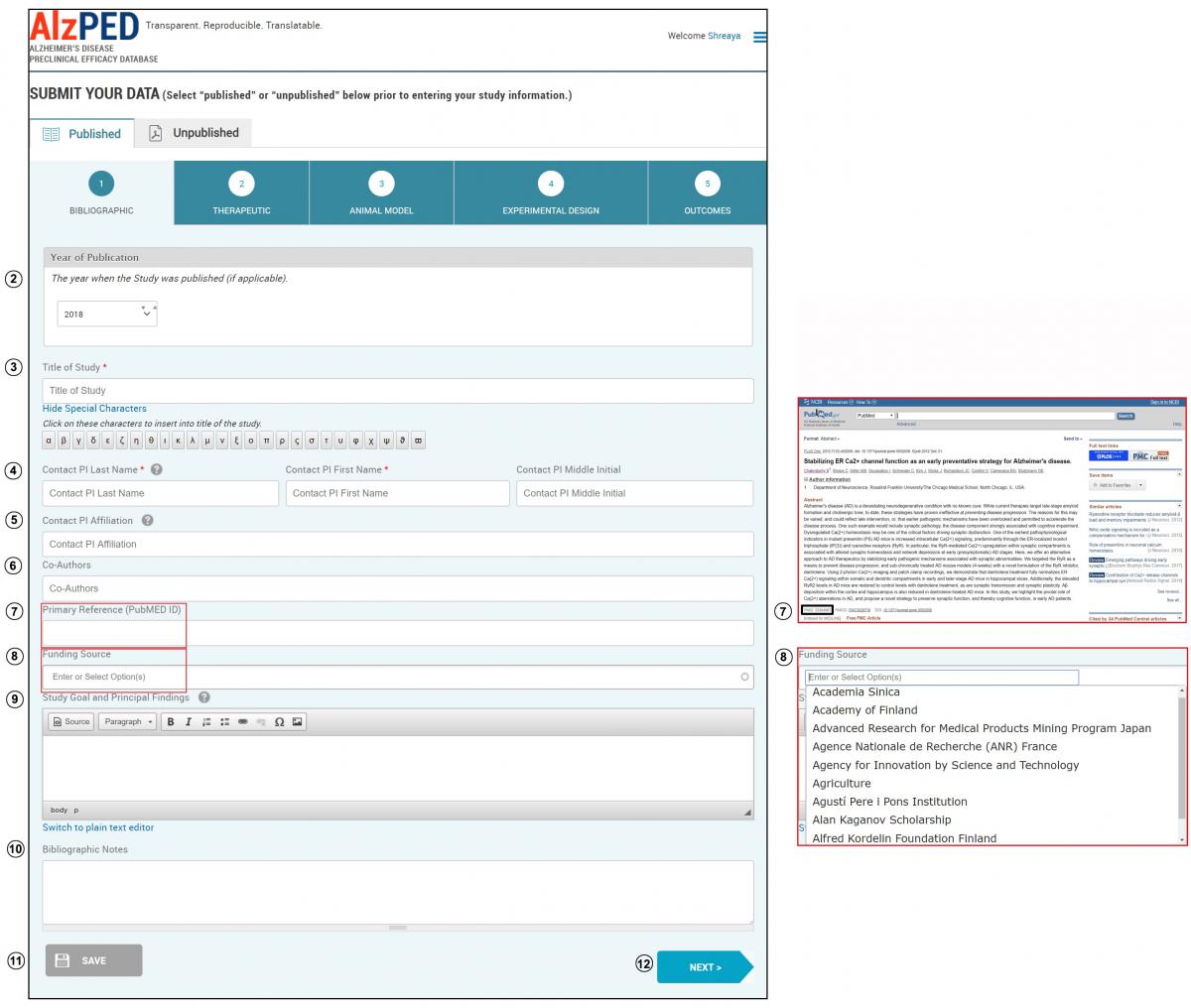
- Once saved, your research article will be assigned an AlzPED ID number (APID) that you can use to find your article once it is published to the AlzPED database.

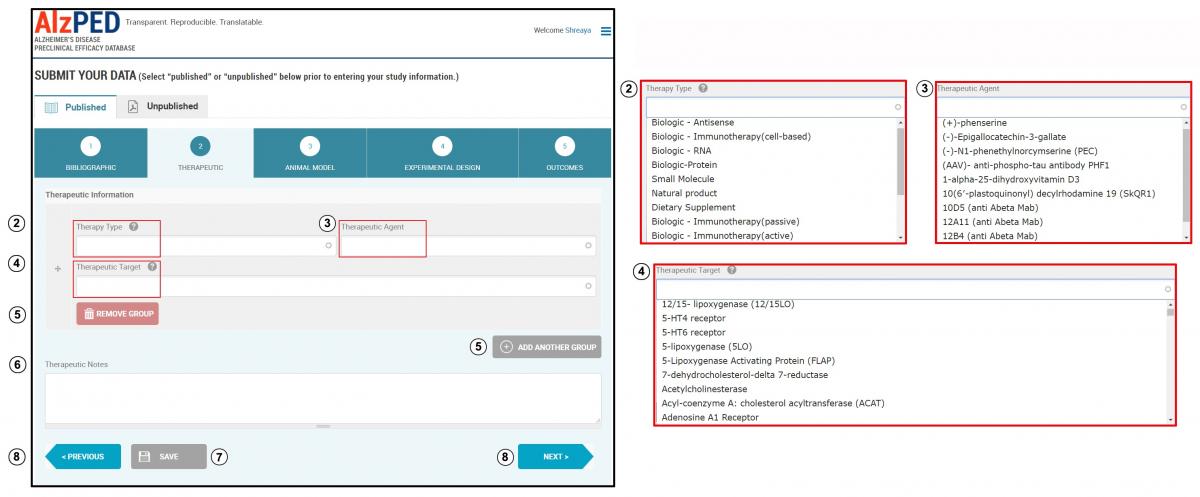
SECTION 2 – THERAPEUTIC
- Complete the fields in this section shown below. Steps 2 – 8 provide detailed instructions about each field.
- Therapy Type: Enter the type of agent based on source (natural product or synthetic molecular structure – example: small molecule, biologic), chemical nature (example: protein, peptide, RNA, DNA, hormone) or mechanism of action (example: biologic – hormone, biologic – immunotherapy). Select the appropriate therapy type from the drop down menu.
- Therapeutic Agent: Enter the name of the therapeutic agent being tested for Alzheimer’s disease-relevant therapeutic effect. Select the appropriate therapeutic agent from the drop down menu or enter the name of the therapeutic agent if it does not appear within the list.
- Therapeutic Target: Enter the name of the molecular or cellular entity through which the therapeutic agent mediates its therapeutic effects. Select the appropriate therapeutic target from the drop down menu or enter the name of the therapeutic target if it does not appear within the list. If the therapeutic agent has multiple targets, select “Multi Target” from the drop down menu.
- If more than one therapeutic agent is used, add additional agents using the “Add Another Group” tool. Remove a therapeutic agent, use the “Remove Group” tool. You must enter at least one therapeutic type, agent and target in this section.
- Therapeutic Notes: Use this space to enter any additional information or unique features related to the therapeutic agent used in the research article.
- Save this section and move to the next.
- You can switch between sections using the “Previous” and “Next” buttons or clicking on the tab for that section.
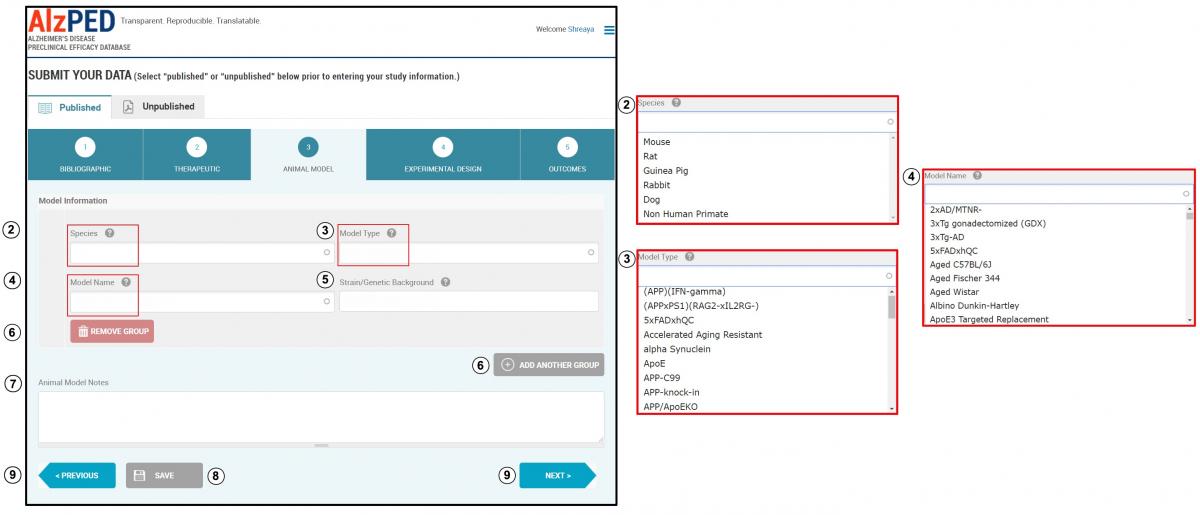
SECTION 3 – ANIMAL MODEL
- Complete the fields in this section shown below. Steps 2 – 9 provide detailed instructions about each field.
- Species: Enter the species of animal(s) used in the research article (example: mouse, rat, non-human primate). Select the appropriate species from the drop down menu.
- Model Type: This refers to the animal model developed using the human gene or homolog believed to be causative or contributing to the neuropathological and clinical phenotypes associated with Alzheimer’s disease (example: APP, APPxPSEN1, hTau). Excluded are animal models with genetic manipulations that have limited clinical relevance to Alzheimer’s disease (example: knock out models of APP, PSEN1, PSEN2 or Tau). Select the appropriate model type from the drop down menu or enter the model type if it does not appear within the list.
- Model Name: This refers to the designation given to the model by the scientific community (example: Tg2576, 3xTg, 3xFAD). In some models, this designation identifies a specific gene/mutation pairing associated with Alzheimer’s disease phenotypes (example: APPswePSEN1d9). Select the appropriate model name from the drop down menu or enter the model name if it does not appear within the list.
- Strain/Genetic Background: This refers to the genetic background of the animal model upon which the human Alzheimer’s disease gene or homolog resides (example: C57BL/6 x C3HF2).
- If more than one animal model is used, add additional models using the “Add Another Group” tool. Remove an animal model, use the “Remove Group” tool. You must enter at least one animal model in this section.
- Animal Model Notes: Use this space to enter any additional information or unique features related to the animal model used in the research article.
- Save this section and move to the next.
- You can switch between sections using the “Previous” and “Next” buttons or clicking on the tab for that section.
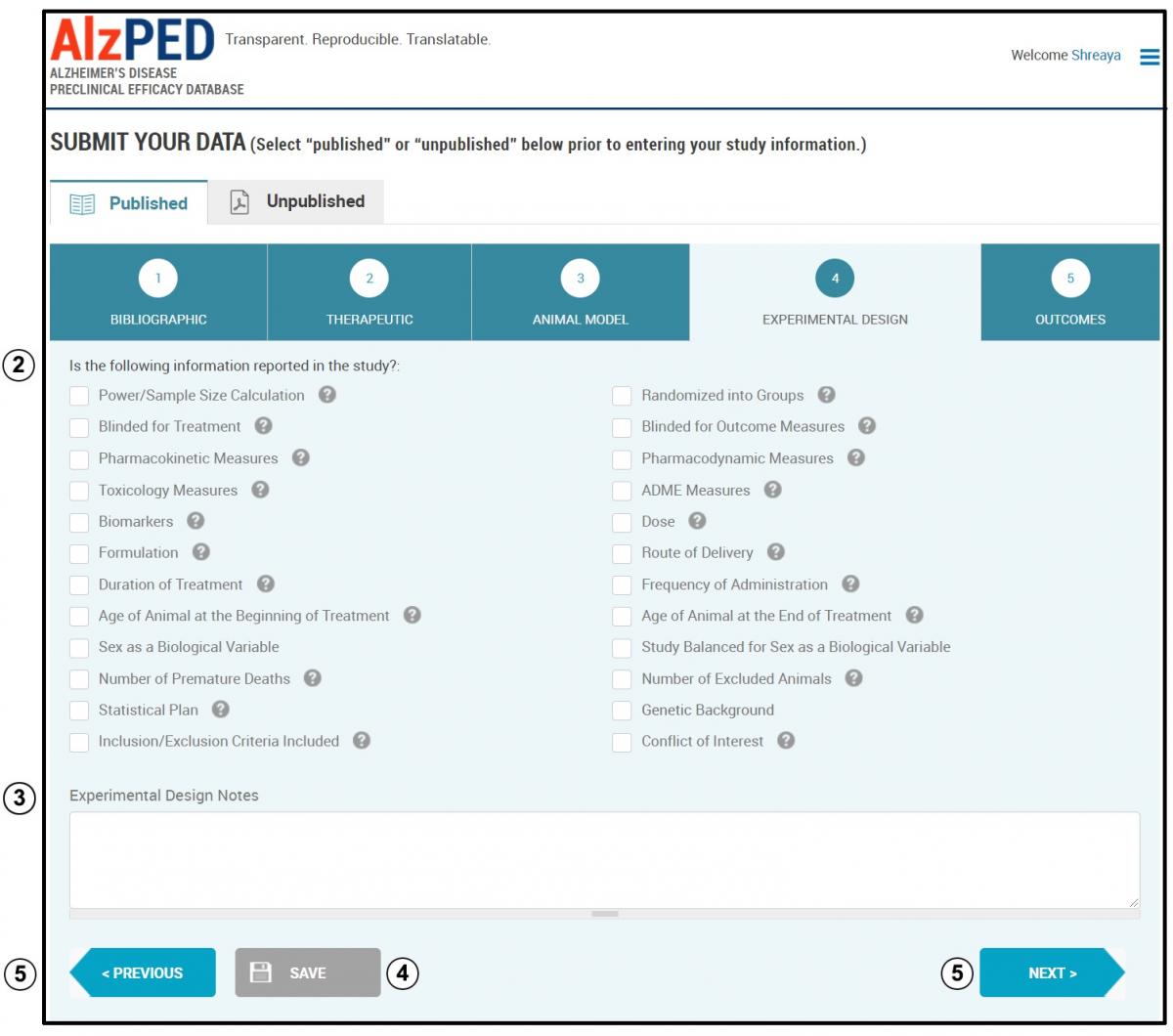
SECTION 4 – EXPERIMENTAL DESIGN
- Complete the fields in this section shown below. Steps 2 – 5 provide detailed instructions about each field.
- Indicate whether your research article includes the experimental design elements listed on this page by placing a check mark in the box adjacent to each element that can be identified within your research article. See the Tool Tips
 for clarifications of terms and definitions.
for clarifications of terms and definitions. - Experimental Design Notes: Enter any additional information relevant to the experimental design described in the research article.
- Save this section and move to the next.
- You can switch between sections using the “Previous” and “Next” buttons or clicking on the tab for that section.
SECTION 5 – OUTCOMES
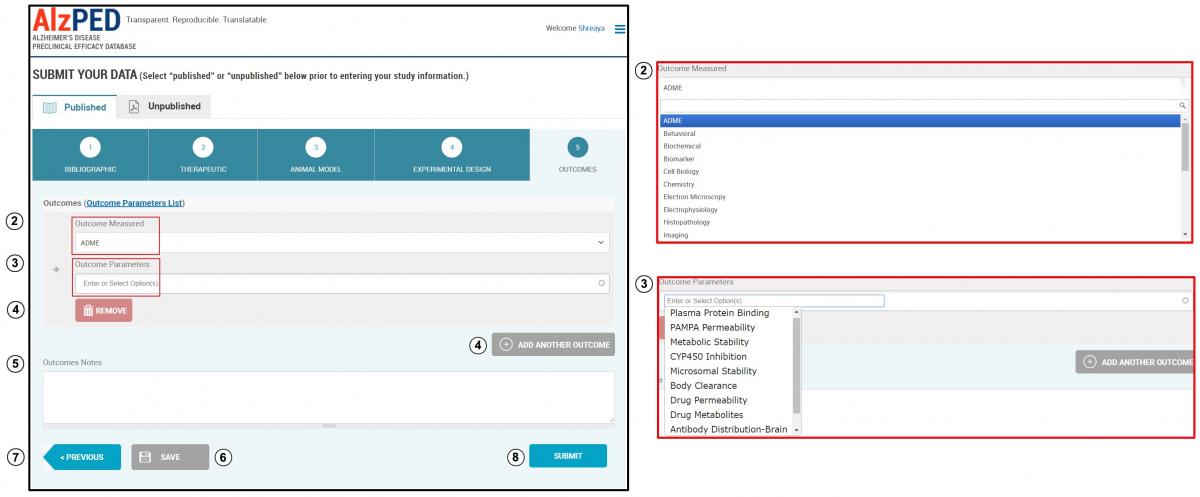
- Complete the fields in this section shown below. Steps 2 – 8 provide detailed instructions about each field.
- Outcome Measured: There are 20 outcome measures included in this list. Select the appropriate outcome measured from the drop down menu.
- Outcome Parameters: Each outcome measure consists of a list of outcome parameters. For a comprehensive list of outcome measures and associated parameters, please follow the link to the “Outcome Parameters List”. This list is downloadable as a CSV file. Select from this list, options that best describe the experimental parameters utilized in your research article using the drop down menu. Add outcome parameters that do not appear within the list.
- Additional outcome measures can be added using the “Add Another Outcome” tool. Remove outcomes using the “Remove” tool.
- Outcome Notes: Use this space to enter any additional information related to the outcomes reported in the research article.
- Save this section.
- Review all of your data for completeness and accuracy. Use the “previous” and “next” buttons to review each section or clicking on the tab for that section.
- Click on the “Submit” button to submit your study Please note: once you submit your data, you cannot make changes until the curator has reviewed the citation. The curator will contact you with any questions or concerns, prior to adding the citation to the AlzPED database.
PREPRINT STUDY - How to Enter Data
MINIMUM REQUIREMENTS FOR SUBMITTED STUDY TO BE CONSIDERED FOR REVIEW AND PUBLICATION
- All sections must be completed. Enter all relevant information you would include if you were submitting the study to a journal for peer review and publication.
- The study must include information on at least one Therapeutic Agent and one Animal Model. Each field in the Therapeutic Agent and Animal Model sections must be completed.
- Review the Experimental Design section carefully and select all areas of Experimental Design for which you have information to report for the study. At minimum, information for the following experimental design elements must be provided: dose and formulation of the therapeutic agent used, route of delivery, duration of treatment and frequency of administration, sex, genetic background and age of the animals used.
- Review the 7 categories in the Resource Authentication Table in the Experimental Design section carefully and provide the full descriptive name of the resource used in the research article so that it can be identified and linked with its description in the report. Include the company, manufacturer or individual that provided the resource or where the resource can be obtained or purchased.
HOW TO ENTER DATA
Select the “Preprint” tab and complete the 5 data sections: BIBLIOGRAPHIC, THERAPEUTIC, ANIMAL MODEL, EXPERIMENTAL DESIGN and OUTCOMES, following the detailed instructions provided for each section. For explanations about the fields in each section follow the provided Tool Tips (?).
You may save information within each section (complete or incomplete), to move onto the next section. You can return to the previous section any time before you upload your data to AlzPED. Please note: once you submit your data, you cannot make changes until the curator has reviewed the citation. The curator will contact you with any questions or concerns, prior to adding the citation to the AlzPED database. If you have any questions, please Contact Us.
SECTION 1 – BIBLIOGRAPHIC
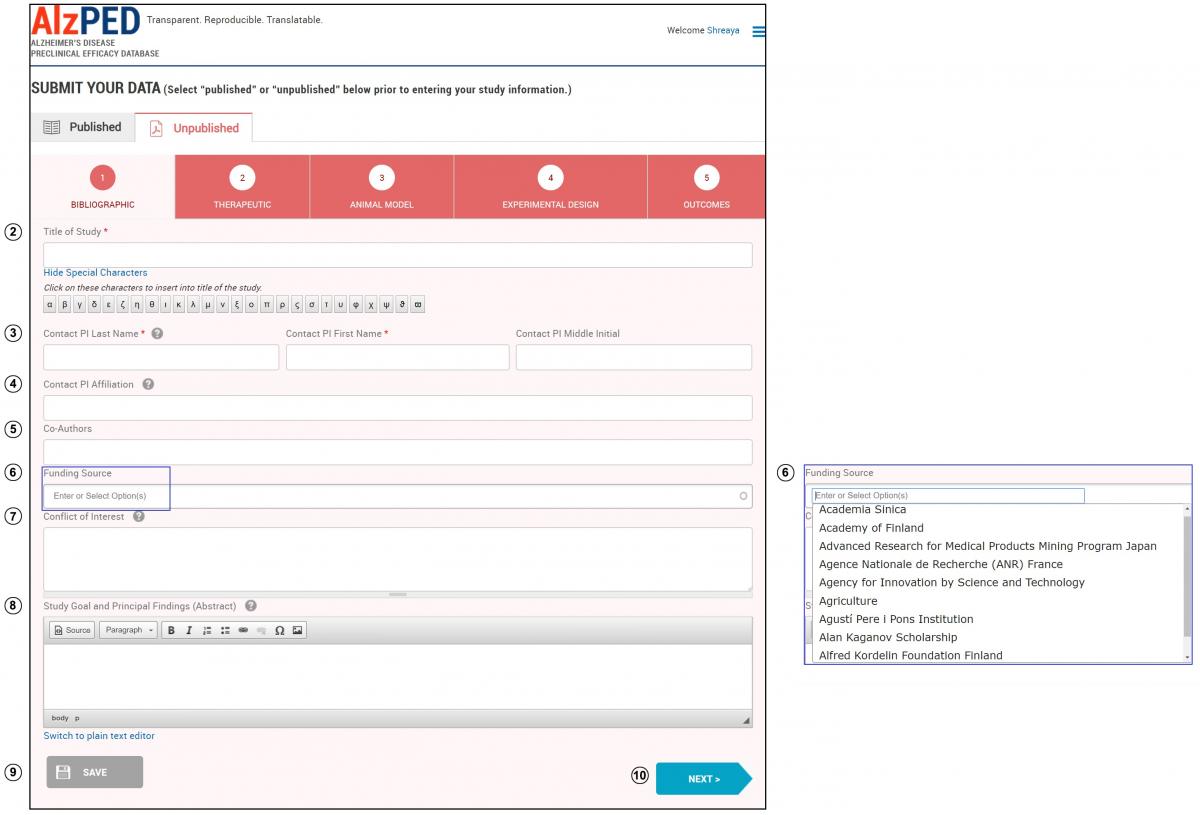
- Complete the fields in this section shown below. Steps 2 – 10 provide detailed instructions about each field.
- Title of Study: Enter the full title of the research article. Click on the “Show Special Characters” link for a list of special characters to include in the title, if required. Contact us if you want to use characters not shown in the tool.
- Contact PI Information – Last Name, First Name, Middle Initial: Enter the name of the primary point of contact (corresponding or senior author) for the research article. If the contact PI formally goes by a middle name, then an initial can be entered in the “First Name” field and a full name can be entered in the “Middle Initial” field.
- Contact PI Affiliation: Enter the Department, School, Institution, City, Region or State, and Country for the contact PI.
- Co-Authors: Enter the names of all co-authors for the research article.
- Funding Source: Enter any financial sources that supported the research. Select one or more funding sources from the drop down menu or enter the funding source if it does not appear within the list.
- Conflict of Interest: Describe in a statement if investigators conducting research do or do not have affiliations with any entity with any financial interest that could affect the design, conduct or reporting of the research. If there are none, then state “There are no conflicts of interest reported by the investigator(s)”.
- Study Goal and Principal Findings: Enter the primary study goal(s), and the overall outcomes found, and the information learned from the study. You can enter your research article abstract in this space.
- Save this section. You will not be able to access other sections until you save the information you have entered in this section.
- Click the “Next” button to move on to the next section. Alternatively, you can complete the remaining sections in any order by clicking on the tab for that section.
- Once saved, your research article will be assigned an AlzPED ID number (APID) that you can use to find your article once it is published to the AlzPED database.

SECTION 2 – THERAPEUTIC
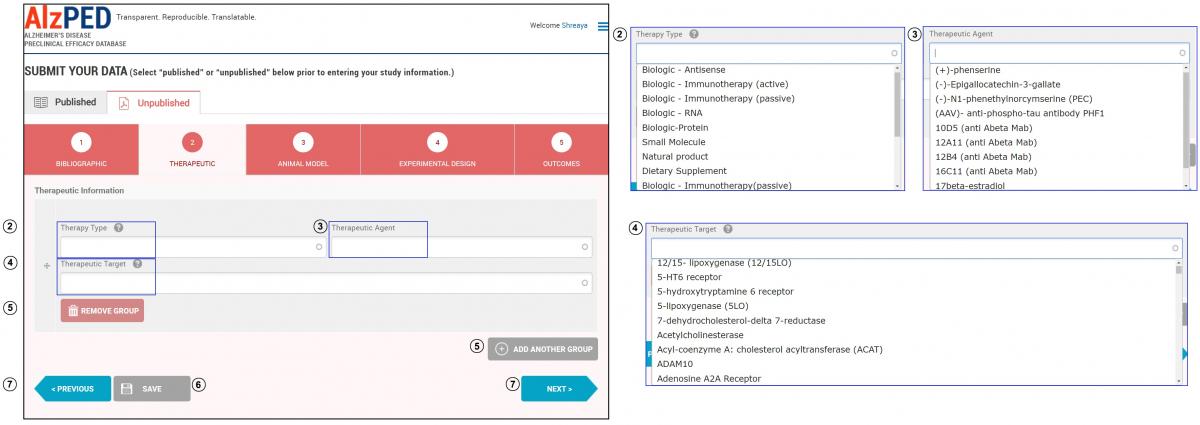
- Complete the fields in this section shown below. Steps 2 – 7 provide detailed instructions about each field.
- Therapy Type: Enter the type of agent based on source (natural product or synthetic molecular structure – example: small molecule, biologic), chemical nature (example: protein, peptide, RNA, DNA, hormone) or mechanism of action (example: biologic – hormone, biologic – immunotherapy). Select the appropriate therapy type from the drop down menu.
- Therapeutic Agent: Enter the name of the therapeutic agent being tested for Alzheimer’s disease-relevant therapeutic effect. Select the appropriate therapeutic agent from the drop down menu or enter the name of the therapeutic agent if it does not appear within the list.
- Therapeutic Target: Enter the name of the molecular or cellular entity through which the therapeutic agent mediates its therapeutic effects. Select the appropriate therapeutic target from the drop down menu or enter the name of the therapeutic target if it does not appear within the list. If the therapeutic agent has multiple targets, select “Multi Target” from the drop down menu.
- If more than one therapeutic agent is used, add additional agents using the “Add Another Group” tool. Remove a therapeutic agent, use the “Remove Group” tool. You must enter at least one therapeutic type, agent and target in this section.
- Save this section and move to the next.
- You can switch between sections using the “Previous” and “Next” buttons or clicking on the tab for that section.
SECTION 3 – ANIMAL MODEL
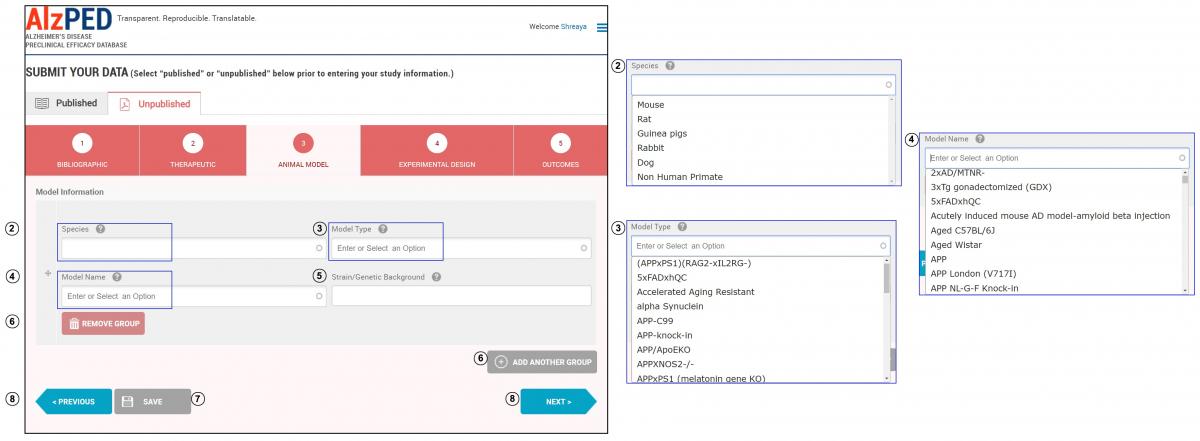
- Complete the fields in this section shown below. Steps 2 – 8 provide detailed instructions about each field.
- Species: Enter the species of animal(s) used in the research article (example: mouse, rat, non-human primate). Select the appropriate species from the drop down menu.
- Model Type: This refers to the animal model developed using the human gene or homolog believed to be causative or contributing to the neuropathological and clinical phenotypes associated with Alzheimer’s disease (example: APP, APPxPSEN1, hTau). Excluded are animal models with genetic manipulations that have limited clinical relevance to Alzheimer’s disease (example: knock out models of APP, PSEN1, PSEN2 or Tau). Select the appropriate model type from the drop down menu or enter the model type if it does not appear within the list.
- Model Name: This refers to the designation given to the model by the scientific community (example: Tg2576, 3xTg, 3xFAD). In some models, this designation identifies a specific gene/mutation pairing associated with Alzheimer’s disease phenotypes (example: APPswePSEN1d9). Select the appropriate model name from the drop down menu or enter the model name if it does not appear within the list.
- Strain/Genetic Background: This refers to the genetic background of the animal model upon which the human Alzheimer’s disease gene or homolog resides (example: C57BL/6 x C3HF2).
- If more than one animal model is used, add additional models using the “Add Another Group” tool. Remove an animal model, use the “Remove Group” tool. You must enter at least one animal model in this section.
- Save this section and move to the next.
- You can switch between sections using the “Previous” and “Next” buttons or clicking on the tab for that section.
SECTION 4 – EXPERIMENTAL DESIGN
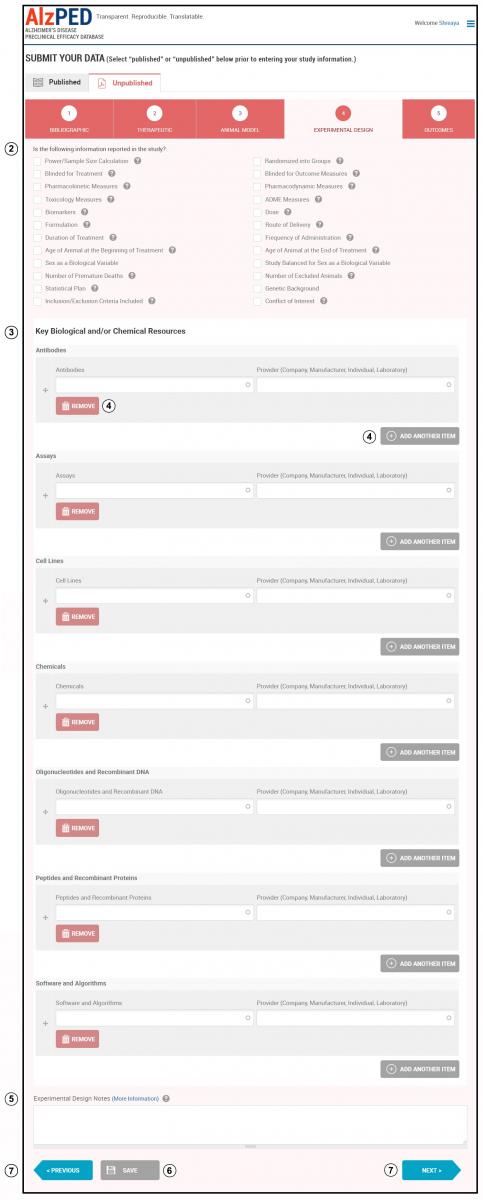
- Complete the fields in this section shown below. Steps 2 – 7 provide detailed instructions about each field.
- Indicate whether your research article includes the experimental design elements listed on this page by placing a check mark in the box adjacent to each element that can be identified within your research article. See the Tool Tips
 for clarifications of terms and definitions.
for clarifications of terms and definitions. - Authentication of Key Biological and/or Chemical Resources: Use the table to provide the full descriptive name of the resource used in the research article so that it can be identified and linked with its description in the report. Include the company, manufacturer or individual that provided the resource or where the resource can be obtained or purchased. Chemicals such as standard culture media and standard buffer solutions do not need to be listed in the table. Skip the sections that are not relevant to your research. The Key Biological and/or Chemical Resources table includes the following 7 categories with examples provided for each category: Antibodies (example: mouse monoclonal anti-amyloid beta 4G8 from Millipore), Assays (example: CellTiterGlo from Promega), Cell Lines (example: HeLa from ATCC), Chemicals (example: picrotoxin from Sigma-Aldrich), Oligonucleotides and Recombinant DNA (example: primer RyR1 (F): 5’-TCTTCCCTGCTGGAGACTGT-3’ from Invitrogen), Peptides and Recombinant Proteins (example: Human TGF beta from Novus Biologicals), Software and Algorithms (example: MetaMorph from Molecular Devices).
- If more than one item is used for a resource category, add additional items using the “Add Another Item” tool. Remove an item, use the “Remove Item” tool.
- Experimental Design Notes: Enter any additional information relevant to the experimental design described in your research article.
- Save this section and move to the next.
7. You can switch between sections using the “Previous” and “Next” buttons or clicking on the tab for that section.
SECTION 5 – OUTCOMES
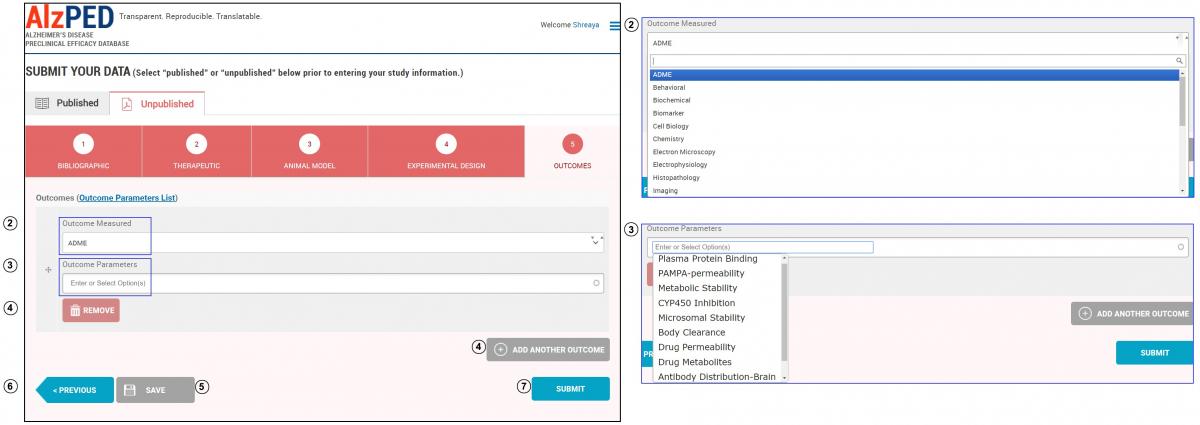
- Complete the fields in this section shown below. Steps 2 – 7 provide detailed instructions about each field.
- Outcome Measured: There are 21 outcome measures included in this list. Select the appropriate outcome measured from the drop down menu.
- Outcome Parameters: Each outcome measure consists of a list of outcome parameters. For a comprehensive list of outcome measures and associated parameters, please follow the link to the “Outcome Parameters List”. This list is downloadable as a CSV file. Select from this list, options that best describe the experimental parameters utilized in your research article using the drop down menu. Add outcome parameters that do not appear within the list.
- Additional outcome measures can be added using the “Add Another Outcome” tool. Remove outcomes using the “Remove” tool.
- Save this section.
- Review all of your data for completeness and accuracy. Use the “previous” and “next” buttons to review each section or clicking on the tab for that section.
- Click on the “Submit” button to submit your study Please note: once you submit your data, you cannot make changes until the curator has reviewed the citation. The curator will contact you with any questions or concerns, prior to adding the citation to the AlzPED database.
REVIEW PROCESS
- The submission will first be evaluated to confirm that it meets the minimum requirements to be considered for review.
- Any submission that does not meet the minimum requirements for review will be rejected. Resubmissions will be allowed provided the minimum requirements for review are met.
- Submissions that meet the minimum requirements for review will be reviewed by two NIA experts. This initial review will take 2 weeks from the date of submission. When the initial review is complete, the submitted report will be returned to you with a request for additional information and instructions on how to submit the requested information. Submitted additional information will also be reviewed by two NIA experts within 2 weeks from date of submission.
- Once accepted, the preprint report will be published on the Synapse-Sage Bionetworks open science knowledge portal.
- A citable DOI will be emailed to you, that you can cite in your grant applications, publications and resume.
 for clarifications of terms and definitions.
for clarifications of terms and definitions.